Introduction
A well-optimized plan for ion radiotherapy can frequently be attained using no more than four beam-entry angles achieved through the rotation of the gantry, rotation of the patient positioning table, or a combination of both. The selection of these angles is pivotal for achieving the prescribed target dose while minimizing the dose to the organs at risk (OARs) [1, 2]. However, the practical implementation of a carbon-ion rotational gantry is challenging, with dimensions and weight double those of proton gantries, currently limited to institutions in Germany and Japan [3-7]. With the advancement of superconducting magnet technology, the first superconducting carbon-ion gantry was installed at the National Institutes for Quantum Science and Technology (QST), which reduced the weight of the gantry at the Heidelberg Ion-Beam Therapy Center (HIT) from 600 to 300 tons [5]. Toshiba has increased the superconducting magnetic field strength to 3.50 T to develop a more compact carbon ion gantry. This gantry is currently in the commissioning phase at Yamagata University and Yonsei University Health System. The size of the system has been reduced to two-thirds that of the first superconducting gantry in QST [8]. Radiation protection in carbon-ion radiotherapy is crucial because of the necessity for high-energy therapeutic carbon-ion beams for treating deep-seated tumors [9-12]. This importance is amplified when considering the rotating gantry for carbon-ion therapy. Given the substantial size and cost of carbon ion rotational gantries, Shanghai Proton and Heavy Ion Center (SPHIC), as most institutions globally, has not yet adopted this technology [13]. SPHIC currently operates four proton and heavy ion therapy rooms, catering to diverse tumor locations, depths, and positioning requirements, including three horizontal beamline treatment rooms and one 45° beamline treatment room.
Carbon ion radiotherapy, distinguished by its superior physical dosimetry compared to that of conventional radiation and augmented by the biological advantages of carbon ion beams, holds promise for better preservation of healthy tissue and increased tumor control [14]. Consequently, it has been increasingly employed in the treatment of patients with compromised pulmonary or cardiac function [15-17]. Given the significant correlation between the mean lung dose and risk of radiation pneumonitis, it is imperative to minimize the mean lung dose [18, 19]. The selection of appropriate beam angles is crucial in achieving this objective [20]. Because particle therapy typically involves fewer beam-entry angles than that of conventional radiation, providing freedom in selecting beam angles is crucial from a hardware standpoint.
Usage challenges arise because the fixed-angle beamline and limited roll rotation of the treatment couch hinder optimal treatment for certain patients. In addition, the 45° beamline treatment room faces significant patient waiting times, impacting subsequent treatment processes. Recently, a six-degree-of-freedom treatment chair (6DTC) was designed and installed at our center [2, 21]. However, limited by the absence of an upright CT, the 6DTC can only treat patients with head and neck cancers [1, 22]. To address constraints in treating lung and abdominal tumors, a clinical solution has been developed: a rotational pod. This pod enables patients to be fixed and rotated by 360 degrees (180 clockwise and 180 counterclockwise), simulating the effect of a rotational gantry, thereby ensuring the necessary beam angles and desired dose distribution.
The concept of treatment pods, pioneered by the Paul Scherrer Institute in Switzerland [23], utilizes pi-meson beams directed at tumors through magnets, allowing three-dimensional movement of patients during treatment. Loma Linda University in the United States employs advanced technology [24], utilizing a white semi-cylindrical pod filled with a liquid polyurethane mixture that expands into a solid foam for personalized fixation. In Japan, QST incorporates respiratory gating technology into a rotational pod [25], enabling ±20-degree rotation. The University of Sydney integrates an Elekta linear accelerator and a Patient Rotational System [26] to achieve submillimeter accuracy.
Despite these advances, there is currently a paucity of literature providing detailed clinical data on patients treated with pods. This study aims to fill this gap by presenting comprehensive clinical trial data from patients treated with rotational pods at our center. Specifically, this study aims to investigate the dosimetric benefits of the increased flexibility of beam angle selection provided by pods for lung cancer patients through pre-dosimetry analysis. Additionally, it will validate the dosimetric advantages by examining clinical treatment data from pod patients. The positional accuracy of the pods and the impact of settlement on the stability of tumors and OARs are also calculated and evaluated.
Materials and methods
Structure of the pod
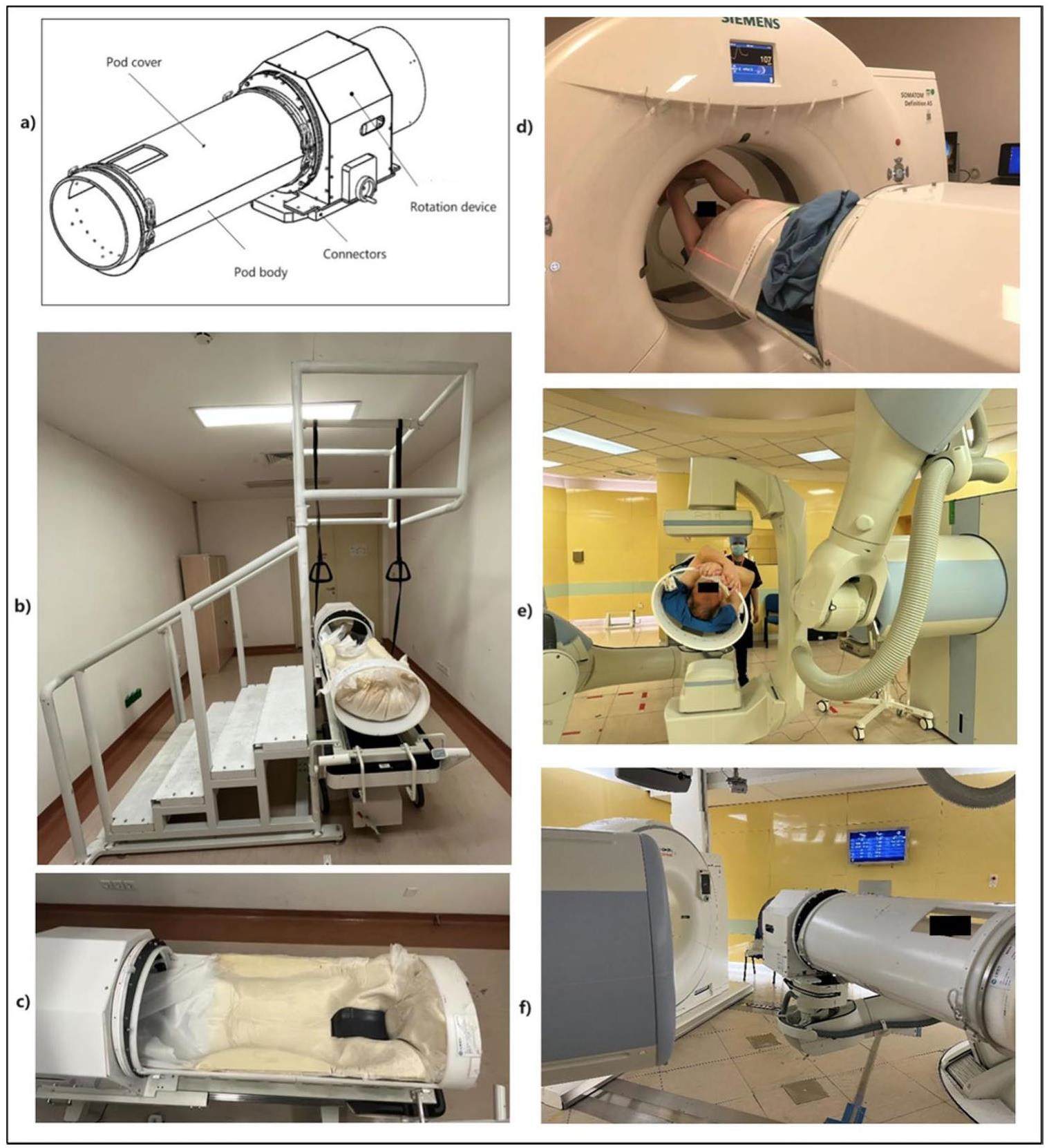
The rotational pod comprises four key components: the pod body, pod cover, rotation device, and connectors, as depicted in Fig. 1. Patients can recline inside the rotating treatment pod using a staircase and handrails. The rotating treatment pod can then be transported using a transfer cart and maneuvered into the CT positioning room or treatment room, connecting it with the current clinical 6-DOF robotic arm via connectors. The rotational robotic arm allows for the necessary adjustments and displacements of the rotating pod within the treatment space, thereby minimizing collisions with other preexisting equipment in the treatment room. The rotation device enables 360-degree roll rotation of the pod body and features a locking mechanism. The pod cover facilitates patient entry and closes securely when the patient remains inside. A specifically designed facial opening in the pod cover ensures that the patient's face is fully exposed, thereby fostering improved communication with the physicians and therapists upon entering the rotating treatment pod.
Pre-dosimetry study
Before initiating the clinical trial, a preliminary dosimetry study was conducted to identify suitable indications for pod use. The average lung dose is a critical OAR in lung cancer radiotherapy. Typically, selecting a path that traverses the shortest distance through the lungs can minimize the average lung dose [27]. However, our facility is constrained to beamlines with angles of 90 and 45 degrees, which limits the options for the beam-entry directions. The application of a pod introduces greater flexibility in the selection of entry angles. In this study, 11 patients who demonstrated a reduced path through the lung when the couch was rotated at a certain angle were selected from those who had previously undergone carbon ion radiotherapy at our center. The patients' CT scans and structures were imported into custom-made software and rotated using a rigid transformation method to the angle at which the beam path through the lung was minimized, creating a synthetic CT. The pod simulation plans were optimized using the same prescription and dose limits as those for the treatment plans with the conventional treatment couch. Then, the target coverage and dose to the OARs were compared between the couch-based and pod simulation plans.
Immobilization and CT Simulation
During multidisciplinary team (MDT) discussions, physicians and physicists assess whether a patient is potentially suitable for rotational pod treatment based on factors such as tumor location, surrounding normal tissue structures, beam path, and other comprehensive considerations. The treatment angles for the rotational pod are determined based on imaging data. After the MDT discussion, patients undergo immobilization following respiratory training [15, 28]. A low-density foam cradle secures the patient's back, utilizing a thermoplastic mask for fixation when the pod rotation angle is ≤30° and employing a pod cover with foam filling when the angle exceeds 30°. Following a supine 4DCT scan, the pod is rotated to the treatment angle for another CT scan [29]. The parameters of both CT scans are identical. The slice thickness is set to 3 mm. A subsequent scan is performed after 20 min at the treatment angle. During this 20-min interval, the patient remains at the treatment angle, simulating the actual treatment. A respiratory gating system (AZ-733V, Anzai Medical, Japan) was used to mitigate the effects of respiratory motion [30].
Treatment Planning
For each patient, two sets of plans were devised: one utilizing supine CT, and the other incorporating CT with a rotated pod. The treatment plan for the supine CT (hereafter referred to as the supine plan) can employ either a 90-degree or 45-degree beamline. Given that the key objective of the pod development is to alleviate the high occupancy pressure associated with the 45° beamline, the pod plan prioritizes the use of a 90-degree beamline. Depending on the different stages and types of tumors in the patients, different prescriptions of carbon ion irradiation were administered. The relative biological effectiveness (RBE) was calculated using local effect model I [31]. The planning objectives aimed to ensure that at least 95% of the internal gross tumor volume (iGTV) and clinical target volume (CTV) received 99% of the prescription dose while minimizing the OAR dose with no more than four beams. If the dose to the normal lung exceeds the limit, the target coverage is sacrificed [29]. Owing to the absence of a robust planning technique [32] in Syngo (V13, Siemens, Germany), the planning target volume (PTV) was expanded based on plan-specific factors, such as the chosen beam range uncertainty, ranging from 7-15 mm [33, 34]. Patients deemed to require two pod rotation angles during the MDT discussions were immobilized and scanned at both angles. Subsequently, the treatment plans were devised separately based on the respective CT scans. Deformable dose accumulation was performed to assess the total dose to the target and OARs using MIM ((MIM Software, Cleveland, OH, USA). All plans were reviewed by the chief physician. A comparison was made between the target coverage and OAR doses of the two plan types.
Patients were considered eligible for pod treatment based on three criteria: lower OAR dose, comparable target coverage, and no increase in range uncertainty. In cases where both plans demonstrated similar target coverage, OAR doses, and uncertainties, the pod plan was preferred because of its shorter admission waiting time, aligning with the best interests of the patients.
Seven patients were selected to undergo carbon-ion radiotherapy using a pod based on the aforementioned criteria. The characteristics of these seven patients' are shown in Table 1.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Age | 58 | 69 | 54 | 41 | 64 | 48 | 67 |
| Gender | M | M | M | F | M | M | M |
| Weight (kg) | 69 | 56 | 76 | 79 | 63 | 89 | 59 |
| Height (cm) | 169 | 172 | 173 | 168 | 174 | 165 | 168 |
| KPS | 90 | 90 | 90 | 90 | 90 | 90 | 90 |
| Diseasea | NSCLC | LUAD | LSCC | M-MPNST | LSCC | LSCC | LSCC |
| Prescription [Gy(RBE)] | 72/16 | 65/10 | 77/22 | 70.4/16 | 77/22 | 77/22 | 77/22 |
| Ion species | Carbon ion | Carbon ion | Carbon ion | Carbon ion | Carbon ion | Carbon ion | Carbon ion |
| Pod rotation angle (°) | 25 | 45 | 45 | 45+90b | 45 | 35+40° | 30 |
Alignment and Treatment
The treatment procedure was based on the 6DTC treatment protocol established at our center [1]. During the initial fraction of treatment, an in-room CT scan was conducted to ensure minimal tumor or anatomical changes compared to the planning CT scan at the treatment angle. If the CT results were satisfactory, the pod was repositioned to the first treatment angle using the robotic arms. Repositioning of patients or adaptive planning was initiated when anatomical changes occurred between the review and planning CTs [33]. The position was verified using an image-guided radiation therapy (IGRT) system. A set of orthogonal X-ray images was captured and registered with digitally reconstructed radiographs, focusing on the bony anatomy. The positional errors at the initial beam position (PE-B1) were documented, including three translational shifts (lateral, longitudinal, and vertical) and three rotational shifts (iso, pitch, and roll) [1]. Subsequently, the patient's position was adjusted using PE-B1 followed by beam delivery. Upon completion of the first beam delivery, the pod was moved to the second iso rotation angle. The X-ray alignment procedure was repeated to determine the positional errors in the second beam position (PE-B2). After applying PE-B2, a second beam was delivered. This process was repeated if the treatment fraction involves more than two beams. The attending physician assessed and authorized the alignment before each treatment onsite.
Position accuracy analysis
In the treatment of lung cancer patients using the pod, maintaining stability in anatomical structures such as the trachea, thoracic vertebrae, and tumor at the treatment angle is paramount, given the inclined position of the body within the pod. This study conducted a comparative analysis of the anatomical structural stability based on CT images acquired 20 min apart. Physicians delineated key structures, including the iGTV, CTV, trachea, and thoracic vertebrae, in two sets of CT scans obtained around the 20-min mark. After aligning the two images, changes in the anatomical structures were quantitatively assessed using the Dice similarity coefficient (DSC) [35]. The DSC formula is as follows:
Statistical significance was assessed using a two-tailed paired Student's t-test; p < 0.05 was considered statistically significant.
The Institutional Review Board reviewed and approved this study.
Results
Pre-dosimetry study
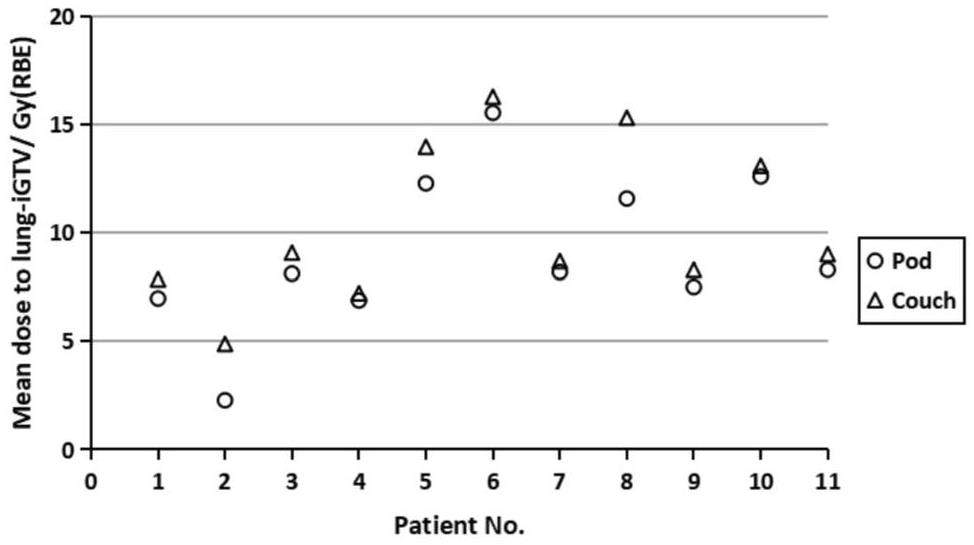
The volumes of the iGTV receiving more than 99% of the prescription dose (V99) were 99.9% ± 0.3% (median ± standard deviation) for the pod plans and 99.9% ±0.8% for the couch plans, and p=0.15 indicates no significant difference. The average dose to the lung-iGTV in the pod plans for the 11 patients decreased by 5–53%, with a p-value less than 0.01 compared to that of the plans using the treatment couch (Figure 2). Moreover, no significant differences were observed in other OARs, such as the heart, esophagus, spinal cord, and trachea.
Planning and Dosimetric Comparison
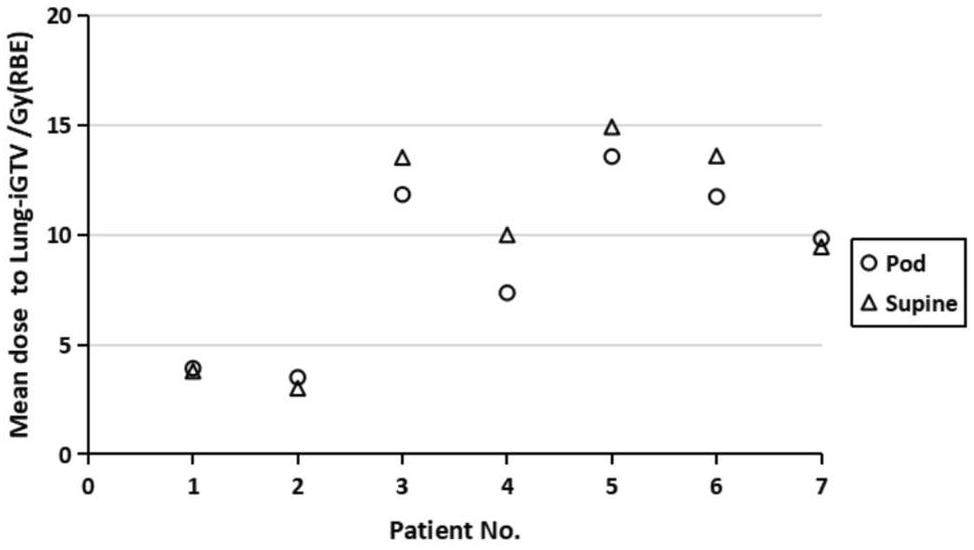
For all seven patients, both the pod and supine plans had comparable target coverage. The V99 of the iGTV for the pod plans were 99.8% ± 7.4% and 99.9% ±3.7% for the supine plans; p=0.31 indicates no significant difference. The V99 of the CTV for the pod plans were 98.8% ± 8.3% and 99.9% ±8.4% for the supine plans; p=0.19 indicates no significant difference. Except for the mean dose to the lung-iGTV, there were no significant differences in the other OARs between the pod and supine plans. The detailed data on the targets and OARs are presented in Table 2. The mean dose to the lung-iGTV is shown in Fig. 3. Notably, for patients numbered 3-6, the dose to the lungs was significantly reduced in the pod plan. While the remaining three patients showed no significant difference in the lung dose, the utilization of the 90° beamline in their cases resulted in a substantial reduction in the patient admission waiting time.
| ROI | Parameter | Pod plan | Supine plan | P value |
|---|---|---|---|---|
| iGTV | V99 (%) | 99.8±7.4 | 99.9±3.7 | 0.31 |
| CTV | V99 (%) | 98.8±8.3 | 99.9±8.4 | 0.19 |
| Lung-iGTV | Dmean [Gy(RBE)] | 9.8 ± 3.7 | 10.0 ± 4.4 | 0.04 |
| Lung-iGTV | V5(%) | 22.4 ± 10.3 | 28.2 ±11.5 | 0.04 |
| Lung-iGTV | V20(%) | 15.8±6.3 | 15.4±8.4 | 0.09 |
| Heart | Dmean [Gy(RBE)] | 4.6±3.5 | 5.9±3.5 | 0.83 |
| Spinal cord | Dmax [Gy(RBE)] | 25.7±13.1 | 28.2±16.5 | 0.48 |
| Trachea | Dmax [Gy(RBE)] | 78.8±26.8 | 78.0±19.3 | 0.72 |
| Esophagus | Dmax [Gy(RBE)] | 78.4 ± 31.5 | 78.4±34.1 | 0.50 |
| Esophagus | Dmean[Gy(RBE)] | 13.2±10.6 | 17.92±17.0 | 0.09 |
Patient alignment
The median treatment duration (from entering to exiting the pod) for the seven patients treated with the pod was 35 min (SD 14 min) per fraction. The positional errors between the beams were recorded and calculated. Figure 4 depicts the deviations in the six directions for all seven patients. The median deviations for the lateral, longitudinal, vertical, ISO, pitch, and roll directions is 0.0 mm ± 5.3 mm, -1.2 mm ± 2.3 mm, -1.1 mm ± 2.7 mm, 0.0° ± 0.6°, -0.1° ± 0.5°, and 0.0° ± 0.8°, respectively.
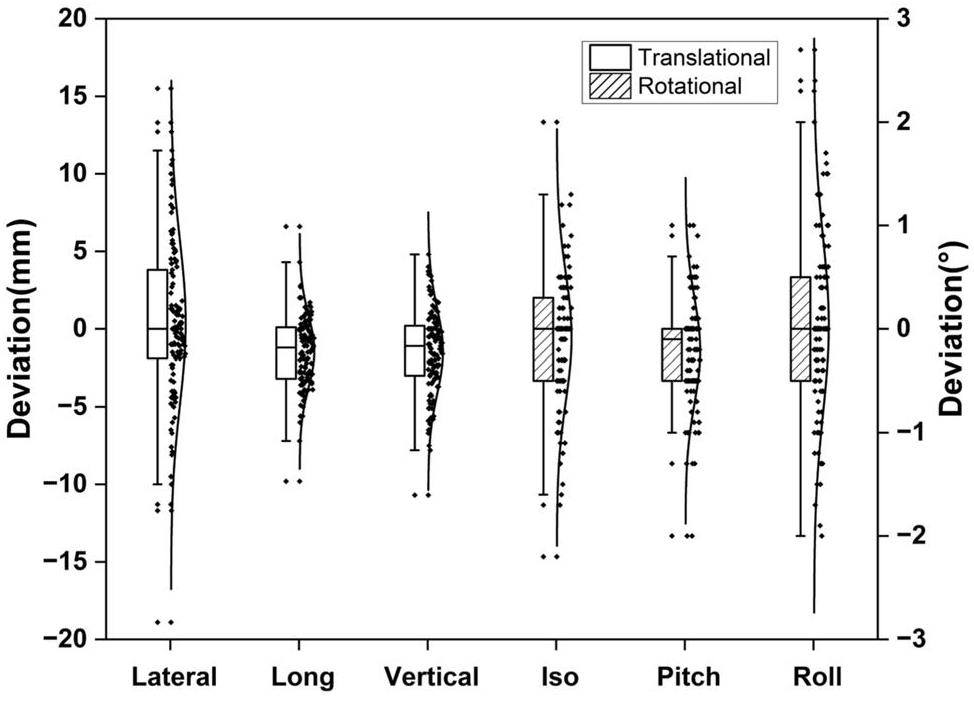
For each fraction, the deviation frequencies in the lateral, longitudinal, and vertical directions within 5 mm were 72%, 95%, and 88%, respectively. For deviations within 10 mm, the frequencies were 93%, 100%, and 99% in the three translational directions, respectively. In the iso, pitch, and roll directions, 91%, 96%, and 87% of the deviations were within 1°, respectively. No deviations exceeding 2° were observed for any of the rotational axes. Notably, these deviations were recorded prior to applying the position correction before the second beam delivery, indicating that they did not affect the precision of the treatment.
Position accuracy analysis
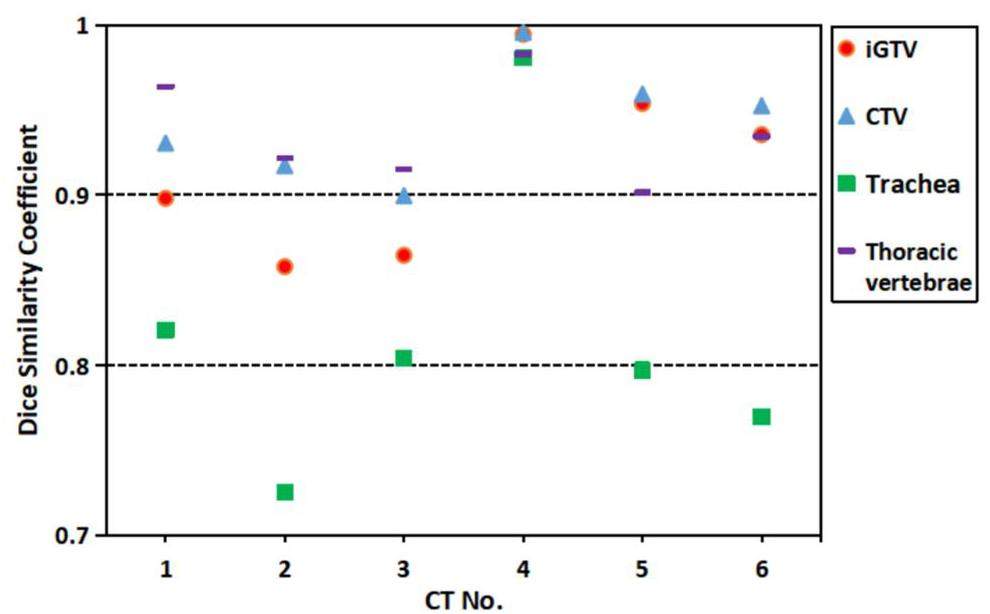
Six pairs of CT images were acquired. The DSC for the iGTV, CTV, trachea, and thoracic vertebrae based on CT images acquired 20-min apart were calculated and recorded.
As shown in Fig. 5, the tumor target and thoracic vertebrae exhibited a high DSC, indicating good stability of these structures. However, the trachea showed a DSC value of approximately 0.8, suggesting potential settling after 20-min of rotation. Nevertheless, a 1-2 mm planning organ-at-risk volume will be expanded during planning, ensuring that its dose remains within safe limits.
Discussion
In this study, we illustrated the efficacy of the pod in achieving target coverage while minimizing doses to OARs and highlighted the challenges associated with treating lung tumors using a rotational pod. Additionally, we presented patient data from a clinical trial and analyzed the position accuracy and stability of anatomical structures.
The pod's capability to simulate the impact of a rotational gantry provides a pragmatic remedy for institutions that lack access to carbon ion rotational gantries. The study revealed a notable reduction in the lung dose in four of the seven patients, suggesting a potential advantage in mitigating radiation exposure to healthy tissues. A lower mean lung dose effectively reduced the risk of radiation pneumonitis [36]. While the remaining three patients did not show dosimetric benefits with the pod plans, the shorter admission waiting time associated with the pod plans aligns with patient-centric considerations. A reduced waiting period for admission may additionally attenuate tumor growth during this interval and diminish the likelihood of treatment replanning.
Patient intra-fractional movements and the rotational accuracy of the robotic arm were the main contributors to the differences between PE-B1 and PE-B2. Sheng et al. validated the submillimeter mechanical accuracy of a robotic arm in our facility [2]. We observed millimeter-level inter-beam movements, with 5–28% of the beams experiencing translational shifts exceeding 5 mm. In Lu's study, 46.7% of the patients exhibited intra-fractional movements exceeding 3 mm [37]. Zhong et al. reported the presence of 5 mm-level systematic and random errors in lung cancer [38]. These results are consistent with the data presented in the present study. This could be attributed to the presence of millimeter-scale motion spaces in patients, even when thermoplastic masks are in place. In addition, the patient's respiratory and cardiac motions contributed to this error. However, it is worth noting that Figure 4 indicates that the error follows a Gaussian distribution, with 72%–95% of the data in the three translational directions falling within 5 mm. Although individual fractions exceeding 5 mm can lead to underdosing of the target or overdosing of OARs, segmentation into 10-22 fractions can reduce the impact of single-fraction errors to some extent. In addition, this millimeter-level inter-beam movement suggests the possibility of relative displacement between the patient, pod, and foam. However, because the pod is designed to be uniform in water equivalence at all angles and depths, and the foam is a low-density material, this error theoretically has a minimal impact on dosimetry. Kanai et al. examined the dosimetric effects of displacement between the patient and the treatment couch when a carbon-ion beam passed through the couch using a rotating gantry. For patients with lung cancer, an average displacement of 4.2 mm was observed; however, dosimetric analysis indicated no significant impact on the target coverage and V20 of the ipsilateral lung[39]. Based on these potential effects, a 7-15 mm margin was expanded to generate the PTV during treatment planning. In addition, position verification at each iso rotation angle is strongly recommended to eliminate this error and ensure accuracy.
The time required for pod treatment is 35 min ± 14 min per fraction, while the treatment couch, also for lung cancer patients, require 21.5 min ± 4 min. As described earlier, the difference between PE-B1 and PE-B2 can sometimes exceed 5 mm, which necessitates position verification before each beam irradiation. This additional step extends the treatment time. However, from the perspective of beamline efficiency, this has a minimal impact because our facility is equipped with four treatment rooms. During position verification, the beamline can be allocated to other rooms, and the treatment can proceed once position correction is completed. Therefore, there was no significant difference in the beam time between the pod patients and those using the treatment couch.
To the best of our knowledge, there is a lack of clinical data on the settling effects of organs when patients shift from an upright to a lying down or tilted position. Importantly, this settling phenomenon is not confined to patients undergoing pod treatment; individuals in the supine or prone positions, transitioning from a standing to a lying-down posture, may also experience potential settling of internal body structures. In this study, DSC was used to evaluate the settling of the tumors, trachea, and vertebrae 20-min after the patients underwent a specific rotational angle. The DSC of these structures, excluding the trachea, were found to be greater than 0.85, which is considered an appropriate threshold for automatic image segmentation [40].
The pod, as a gantry-free solution, can provide more freedom in beam-angle selection when used with a fixed ion beam line compared to the fixed beamline itself. Although it can simulate a 360-degree beam angle, patient stability may be a concern. Although its impact is small, the use of a rotating gantry eliminates the issue of patient stability. We acknowledge this as a disadvantage compared to the gantry. Nevertheless, its low cost makes it a viable option for low- and middle-income countries. The recent trend in upright radiotherapy, facilitated by chairs that can rotate 360 degrees, provides an alternative to rotational gantries [22, 42, 41]. However, this approach is only suitable for treating patients with head and neck lesions in the absence of an upright CT [1]. One notable advantage of the pod is that it eliminates the need for an additional upright CT, distinguishing it from the limitations associated with the current trend of upright radiotherapy. This study has certain limitations, such as a relatively small sample size. However, in the developmental phase of an innovation, studies with small sample sizes can play a pivotal role, and further investigation is required on this topic. Additionally, owing to the lack of real-time KV imaging guidance, it was not possible to obtain patient movement data within individual treatment fields for the analysis and mitigation of patient motion. Further research and technological advancements are required to refine and expand the clinical applications of rotating pods in radiation therapy.
Conclusion
Preliminary clinical application of the rotational pod for lung tumor in fixed ion beam lines presents promising results. This study provides clinical data on the dosimetric considerations, treatment efficiency, and anatomical structural stability associated with rotational pod treatment. However, as an innovative solution to the challenges of ion beam therapy, the rotating pod at SPHIC warrants further exploration and refinement. Future developments could focus on enhancing patient comfort by introducing 3D printing into patient immobilization procedures and establishing robust simulation systems with 4D optimization to further refine treatment plans. Continued research and clinical trials, including those expanding the indications for breast cancer and other malignancies, are essential for validating and optimizing the efficacy of this novel approach.
Clinical implementation of a 6D treatment chair for fixed ion beam lines
. Front. Oncol. 11,Performance of a 6D treatment chair for patient positioning in an upright posture for fixed ion beam lines
. Front. Oncol. 10, 122 (2020). https://doi.org/10.3389/fonc.2020.00122.Commissioning of a fluoroscopic‐based real‐time markerless tumor tracking system in a superconducting rotating gantry for carbon‐ion pencil beam scanning treatment
. Med. Phys. 46(4), 1561 (2019). https://doi.org/10.1002/mp.13403.New technologies for carbon-ion radiotherapy — developments at the National Institute of Radiological Sciences
, QST, Japan. Radiat. Phys. Chem. 162, 90 (2019). https://doi.org/10.1016/j.radphyschem.2019.04.038.Beam commissioning of a superconducting rotating-gantry for carbon-ion radiotherapy
. Nucl. Instrum. Meth. Phy. Res. Sect. A 834, 71-80 (2016). https://doi.org/10.1016/j.nima.2016.07.050.First prospective feasibility study of carbon-ion radiotherapy using compact superconducting rotating gantry
. Br. J. Radiol. 92, 1103 (2019). https://doi.org/10.1259/bjr.20190370.Gantry design for proton and carbon hadrontherapy facilities
.Design and magnetic field measurement of the superconducting magnets for the next-generation rotating gantry
. IEEE. Trans. Appl. Supercond. 32(6), 1-4 (2022). https://doi.org/10.1109/TASC.2022.3160973.Assessment of the induced radioactivity in the treatment room of the heavy-ion medical machine in Wuwei using PHITS
. Nucl. Sci. Tech. 34, 29 (2023). https://doi.org/10.1007/s41365-023-01181-8.Monte Carlo study of the neutron ambient dose equivalent at the heavy ion medical machine in Wuwei
. Nucl. Sci. Tech. 33, 119 (2022). https://doi.org/10.1007/s41365-022-01093-z.Therapeutic techniques applied in the heavy-ion therapy at IMP
. Nucl. Instrum. Meth. Phys. B 269 (7), 664-670 (2011). https://doi.org/10.1016/j.nimb.2011.01.125.Radiation shielding design of a compact single-room proton therapy based on synchrotron
. Nucl. Sci. Tech. 31, 1 (2019).https://doi.org/10.1007/s41365-019-0712-1.Flourish of proton and carbon ion radiotherapy in China
. Front. Oncol. 12,Indications of IMRT, PRT and CIRT for HCC from comparisons of dosimetry and normal tissue complication possibility
. Strahlenther Onkol 198, 361-369 (2022). https://doi.org/10.1007/s00066-021-01854-6.Early stage non-small cell lung cancer treated with pencil beam scanning particle therapy: retrospective analysis of early results on safety and efficacy
. Radiat. Oncol. 14(1), 16 (2019). https://doi.org/10.1186/s13014-019-1216-1.Research progress of heavy ion radiotherapy for non-small-cell lung cancer
. Int. J. Mol. Sci. 23, 4 (2022). https://doi.org/10.3390/ijms23042316.Carbon-ion radiotherapy for non-small cell lung cancer with interstitial lung disease: a retrospective analysis
. Radiat. Oncol. 12(1), 144 (2017). https://doi.org/10.1186/s13014-017-0881-1.Clinical and dosimetric predictors of radiation pneumonitis in a large series of patients treated with stereotactic body radiation therapy to the lung
. Int. J. Radiat. Oncol. Biol. Phys. 85(1), 190 (2013). https://doi.org/10.1016/j.ijrobp.2012.03.041.Single-isocenter versus multiple-isocenters for multiple lung metastases: Evaluation of lung dose
. Radiother. Oncol. 166, 189 (2022). https://doi.org/10.1016/j.radonc.2021.11.030.Optimal beam angle selection and knowledge-based planning significantly reduces radiotherapy dose to organs at risk for lung cancer patients
. Acta. Oncol. 60(3), 293 (2021). https://doi.org/10.1080/0284186X.2020.1856409.Development of an isocentric rotating chair positioner to treat patients of head and neck cancer at upright seated position with multiple nonplanar fields in a fixed carbon-ion beamline
. Med. Phys. 47(6), 2450 (2020). https://doi.org/10.1002/mp.14115.Considerations for upright particle therapy patient positioning and associated image guidance
. Front. Oncol. 12,The PIOTRON: initial performance, preparation and experience with pion therapy
. Int. J. Radiat. Oncol. Biol. Phys. 8(9), 1499 (1982). https://doi.org/10.1016/0360-3016(82)90609-5.LLUPTF: Eleven years and beyond
.Overview of clinical experiences on carbon ion radiotherapy at NIRS
. Radiother. Oncol. 73 Suppl 2,Development and commissioning of a full-size prototype fixed-beam radiotherapy system with horizontal patient rotation
. Med. Phys. 46(3), 1331 (2019). https://doi.org/10.1002/mp.13356.Carbon-ion scanning lung treatment planning with respiratory-gated phase-controlled rescanning: simulation study using 4-dimensional CT data
. Radiat. Oncol. 9, 238 (2014). https://doi.org/10.1186/s13014-014-0238-y.Definitive carbon ion radiotherapy for tracheobronchial adenoid cystic carcinoma: a preliminary report
. BMC. Cancer. 21(1), 734 (2021). https://doi.org/10.1186/s12885-021-08493-1.Preliminary safety and efficacy of proton plus carbon-ion radiotherapy with concurrent chemotherapy in limited-stage small cell lung cancer
. Front. Oncol. 11,Technical and dosimetric aspects of respiratory gating using a pressure-sensor motion monitoring system
. Med. Phys. 33(1), 145 (2006). https://doi.org/10.1118/1.2147743.Accuracy of the local effect model for the prediction of biologic effects of carbon ion beams in vitro and in vivo
. Int. J. Radiat. Oncol. Biol. Phys. 71(3), 866 (2008). https://doi.org/10.1016/j.ijrobp.2008.02.037.Comparison between 4D robust optimization methods for carbon-ion treatment planning
. Nucl. Sci. Tech. 34, 139 (2023). https://doi.org/10.1007/s41365-023-01285-1.Adaptive carbon ion radiotherapy for locally advanced non-small cell lung cancer: Organ-sparing potential and target coverage
. Med. Phys. 49(6), 3980 (2022). https://doi.org/10.1002/mp.15563.Dosimetric rationale and preliminary experience in proton plus carbon-ion radiotherapy for esophageal carcinoma: a retrospective analysis
. Radiat. Oncol. 18(1), 195 (2023). https://doi.org/10.1186/s13014-023-02371-9.Deep learning-aided automatic contouring of clinical target volumes for radiotherapy in breast cancer after modified radical mastectomy
. Front. Phys. 9,External validation of NTCP-models for radiation pneumonitis in lung cancer patients treated with chemoradiotherapy
. Radiother. Oncol. 186,Intra- and inter-fractional liver and lung tumor motions treated with SBRT under active breathing control
. J. Appl. Clin. Med. Phys. 19(1), 39 (2018). https://doi.org/10.1002/acm2.12220.Implementation of single-breath-hold cone beam CT guided hypofraction radiotherapy for lung cancer
. Radiat. Oncol. 9, 77 (2014). https://doi.org/10.1186/1748-717X-9-77.Evaluation of patient positional reproducibility on the treatment couch and its impact on dose distribution using rotating gantry system in scanned carbon-ion beam therapy
. Phys. Med. 57, 160-168 (2019). https://doi.org/10.1016/j.ejmp.2018.12.013.Performance of commercially available deformable image registration platforms for contour propagation using patient-based computational phantoms: A multi-institutional study
. Med. Phys. 45(2), 748 (2018). https://doi.org/10.1002/mp.12737.Upright patient positioning for gantry-free breast radiotherapy: feasibility tests using a robotic chair and specialised bras
. Front. Oncol. 13,Relative thoracic changes from supine to upright patient position: A proton collaborative group study
. J. Appl. Clin. Med. Phys. 24(12),The authors declare that they have no competing interests.


