Introduction
With the increasing demand for functionalization and aesthetics of textiles [1, 2], fluorescent whitening agents have been widely used in textile finishing processes [3]. Owing to the noncovalent binding with textiles [4, 5], fluorescent whitening agents always have low utilization in finishing processes (only 60-70%) [6] and are prone to wash-out in household laundry [5, 6]. The effluents from textile finishing and washing also contain difficult-to-treat fluorescent whitening agents [7, 8], which are harmful to the aquatic ecological environment and subsequently to human health [9, 10]. Also, the wash-out of fluorescent whitening agents from fabrics results in textile colour deepening, reducing its marketing value. Therefore, efforts are necessary to improve the finishing utilization and washing durability of fluorescent whitening agents on fabric.
As a powerful tool, high-energy radiation technology has been used frequently in the field of textile and fiber modification[11-16]. Electron beam is used to treat textile wastewater which is difficult to degrade [17]. A large number of novel functional fabric and fiber materials with superhydrophobicity [18, 19], heavy metal ions and dyes[20] adsorption [21] antibacterial [22], antistatic [23, 24], and photothermal properties [25, 26] have been developed with the assistance of electron beam or gamma-ray irradiation. Recently, our group has found that both disperse and reactive dyes with vinyl groups can be covalently grafted onto fiber surfaces under high-energy radiation, which not only improved dye utilization but also enhanced color fastness [27-31]. As it well known, covalent bonds (3-4 eV of bond energy) of the chains in fibers are easily broken by high-energy radiation (1 - 5 MeV), thus generating free radicals on fibers, which rapidly initiate the graft polymerization of vinyl monomers. The covalent bonds between fibers and graft segments contribute to a permanent functionalization [32]. This is why a novel dyeing method based on high-energy radiation achieves high dye utilization and excellent color fastness. The Chemical Oxygen Demand (COD) of wastewater from this process is so low that it meets direct discharge standards [28, 31]. Besides that, this radiation-induced dyeing method significantly reduced the use of various finishing reagents in textile functional modification and has the characteristics of low energy consumption, easy operation, and fast processing speed [33, 34]. Undoubtedly, this is an efficient and environmentally friendly textile processing method, which could promote the traditional textile industry’s transformation in the direction of green and sustainable development.
Inspired from this, a new vinyl-containing fluorescent whitening agent, (E)-6,6’-(ethene-1,2-diyl) bis (3-((4,6-bis(2-(acryloyloxy) ethoxy)-1,3,5-triazin-2-yl) amino) benzenesulfonic acid), was synthesized using 4,4’-diamino-2,2’-stilbenedisulfonic acid (DSDA), cyanuric chloride (CC), and hydroxyethyl methacrylate (HEA) as starting reagents. For brevity, the new fluorescent whitening agent was named as DCH. The DCH was further used to whiten raw cotton fabric with the assistance of electron beam irradiation. The route is summarized in Fig. 1. FT-IR spectroscopy, X-ray photoelectron spectroscopy (XPS), and energy dispersive spectrometry (EDS) were used to confirm the success in fluorescent whitening of cotton fabric under electron beam irradiation. Standard accelerated washing (AATCC61-2006) and light fastness tests (ISO 105-B02: 2014) were conducted to evaluate the wash-durable and sunlight-durable fluorescent whitening effect. Skin safety of the whitened cotton fibric was tested on adult female New Zealand white rabbits according to the international testing standard (ISO 10993-23: 2021). Furthermore, the wastewater from this electron beam irradiation induced fluorescent whitening process was tested by a COD meter and electrical conductivity meter to assess the content of spent organic matters and salts. This study provided a new method to textile finishing process with low pollution and energy-consumption, superior to the traditional chemical finishing methods, contributing to the transformation, and upgrading of the textile industry.
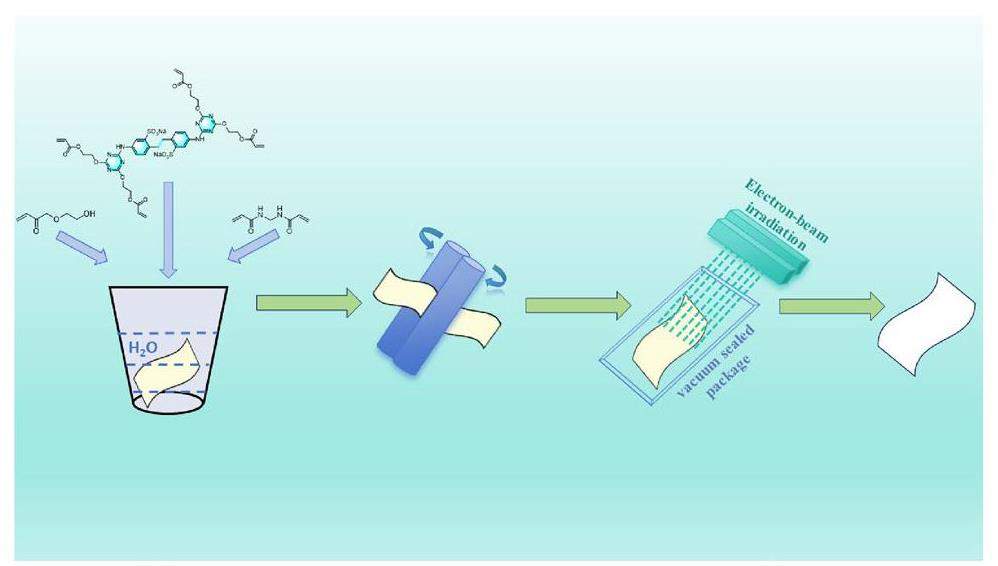
Experimental
Synthesis of DCH
The synthetic route included two steps (Fig. 2). The first step was synthesis of an intermediate, ((6-chloro-1,3,5-triazine-2,4-diyl) bis (oxy)) bis (ethane-2,1-diyl) diacrylate. The details were as follows: Hydroxyethyl methacrylate (HEA, 300 mL), cyanuric chloride (CC, 50 g, 0.271 mol) and 4-hydroxy-2,2,6,6-tetramethyl-piperidinooxy(4H-TEMPO, 15 g) were added to the reaction flask. N, N-diisopropylethylamine (DIPEA, 50 mL, 0.287 mol) was slowly added dropwise in an ice water bath. After this addition, the reaction was stirred for 30 min and DIPEA (50 mL, 0.287 mol) then added. The oil bath was heated to 30 ℃ for 2 h and the reaction monitored by thin layer chromatography (TLC). The organic phase was washed with water until the HEA was washed out, then dried with anhydrous sodium sulfate and ethyl acetate removed in vacuum to obtain the intermediate. Yield: 84%. ESI-MS (CH3OH) m/z: calculated for [M+H]+, (C13H14ClN3O6+) 343.0570, found 344.0621. 1H-NMR (400 MHz, DMSO-d6) δ 6.35 (dd, J = 17.2, 1.6 Hz, 1 H), 6.20 (dd, J = 17.3, 10.3 Hz, 1 H), 5.97 (dd, J = 10.3, 1.6 Hz, 1 H), 4.69–4.52 (m, 2H), and 4.48 – 4.35 (m, 2H). 13C-NMR (101 MHz, DMSO) δ 171.93, 171.85, 165.78, 132.64, 128.36, 67.22, 62.38, 40.58, 40.42, 40.37, 40.22, 40.17, 40.01, 39.96, 39.75, 39.54, and 39.33.
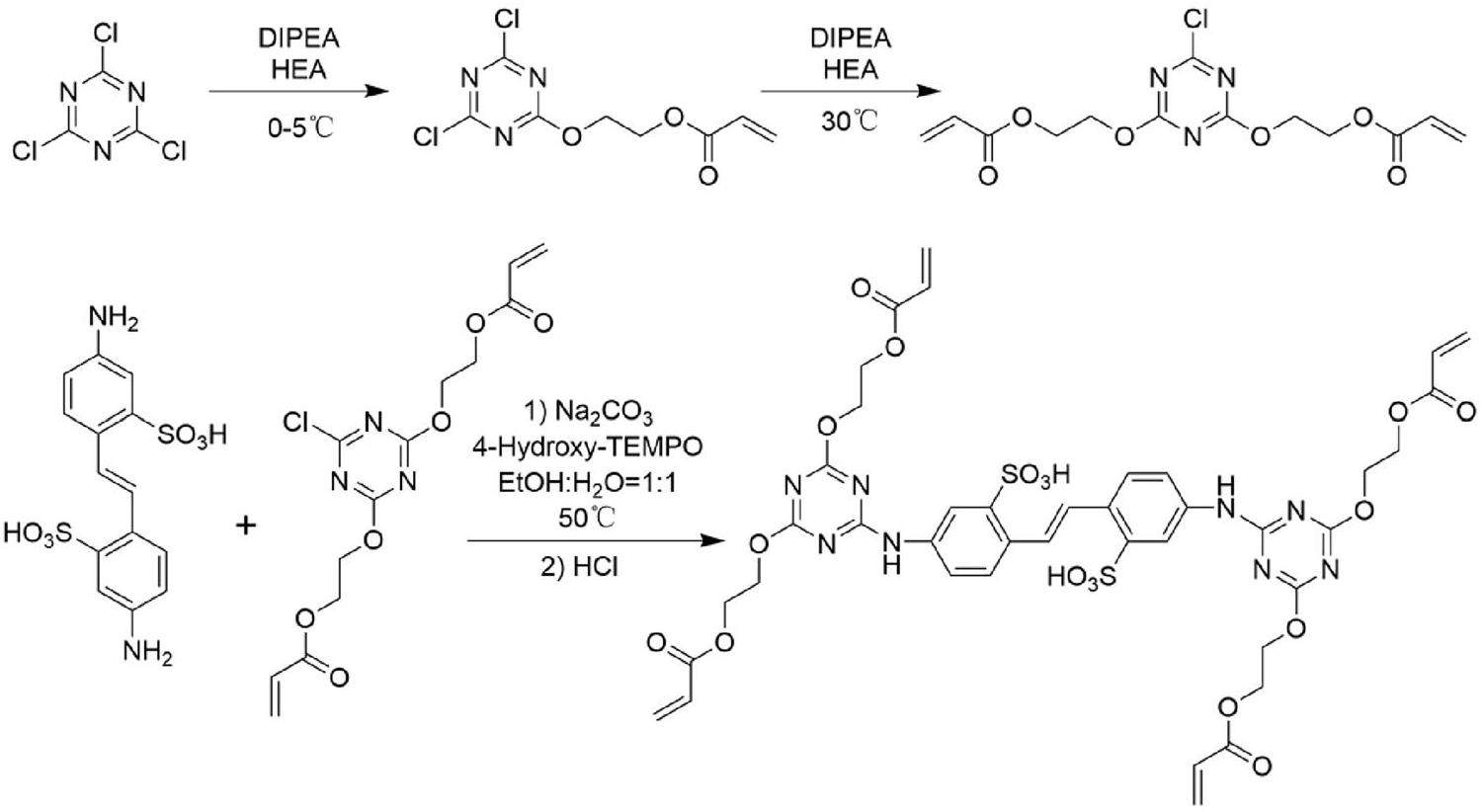
The second step was the reaction between the above intermediate and 4,4’-diamino-2,2’-stilbenedisulfonic acid (DSDA). In the reaction flask, DSDA (0.74 g,0.002 mol), anhydrous sodium carbonate (0.212 g,0.002 mol), water (10 mL), and 4 H-TEMPO (0.086 g) were added and stirred to dissolution. The above intermediate (1.72 g, 0.005 mol) was uniformly dispersed in ethanol (10 mL). After stirring the suspension, it was poured into the reaction flask and heated in the oil bath at 50 °C for 2 h. During the reaction, saturated sodium carbonate solution was added dropwise to control the pH at 7. After the reaction, the HCl solution (0.2 M) was slowly added dropwise until a yellowish solid was precipitated. The solid was filtered, washed with anhydrous ethanol, and vacuum dried to obtain a yellowish solid powder (DCH). Yield: 80.2%. ESI-MS (CH3OH) m/z: calculated for [M-H]− (C40H40N8O18S2−) 984.19, found 984.1794. 1H-NMR (400 MHz, DMSO-d6) δ 10.32 (s, 1H), 7.72 (dd, J = 8.6, 2.4 Hz, 1H), 6.36 (dd, J = 17.3, 1.7 Hz, 1 H), 6.22 (dd, J = 17.3, 10.3 Hz, 1H), 5.97 (dd, J = 10.2, 1.7 Hz, 1H), and 4.74−4.40 (m, 4H).13C-NMR (101 MHz, DMSO) δ 166.06, 165.88, 146.00, 137.31, 132.56, 130.44, 128.49, 126.62, 126.11, 121.12, 119.63, 66.05, 62.85, 40.50, 40.29, 40.09, 39.88, 39.67, 39.46, and 39.25.
Fluorescent whitening of raw cotton fabric by electron beam irradiation
Raw cotton fabric with a size of 25 cm × 15 cm was thoroughly washed with plenty of deionized water and then dried in a drying oven. DCH solutions with certain concentrations were prepared by rigorously stirring at room temperature, with HEA and N, N’-methylenebisacrylamide (MBA) added as assistant ingredients. The dried cotton fabric was fully immersed in the above solution. A cotton fabric ginning machine (Fig. S1) with a pressure of 1 bar and a speed of 2 m min−1 used to press the raw cotton fabric. The DCH solution was squeezed into the gaps of the raw cotton fabric to remove excess solution, such that the DCH was evenly distributed on the raw cotton fabric. The treated cotton fabric sample was vacuum sealed in a plastic bag and irradiated by a self-shielding electron accelerator (1.5 MeV, Shanghai Institute of Applied Physics, CAS, Shanghai, China) for 10 kGy of absorbed dose at room temperature. After irradiation, the treated cotton fabric was taken out and washed in deionized water (60 times to the sample mass) under 90 °C for 3 min. Finally, the washed cotton fabric was dried in a vacuum oven at 60 °C. The spent washing water was collected and tested using COD and electrical conductivity meters. A series of whitened samples were prepared by adjusting the concentration of fluorescent whitening solution (Table 1) and named as FWC fabric, respectively.
| Sample | Mass of DCH (g) | Mass of Na2 CO3 (g) | Mass of HEA (g) | Mass of MBA (g) | Mass of H2O (g) | Concentration |
|---|---|---|---|---|---|---|
| FWC-1 | 0.03 | 0.0032 | 0.03 | 0.02 | 30 | 0.1% |
| FWC-2 | 0.09 | 0.0097 | 0.09 | 0.06 | 30 | 0.3% |
| FWC-3 | 0.15 | 0.0162 | 0.15 | 0.10 | 30 | 0.5% |
| FWC-4 | 0.24 | 0.0259 | 0.24 | 0.16 | 30 | 0.8% |
| FWC-5 | 0.3 | 0.0323 | 0.3 | 0.20 | 30 | 1% |
The utilization rate can be calculated by Eq. (1)
Whiteness Measurements
The fluorescent whitening sample was measured using a Datacolor 800 spectrophotometer (Technical Color Solutions, USA), and untreated cotton fabric was used as the raw cotton fabric. A D65 light source was selected as the test light source. Each sample was measured three times on any surface of the fabric and the average whiteness recorded.
Washing and Light Fastness Test
The fluorescent whitening sample was cut to a size of 50 mm × 150 mm. To evaluate the washing fastness of the whitening cotton fabric, according to the AATCC61-2006 test method, the accelerated washing durability test was carried out using condition 2A and one of the accelerated washing cycles equal to five home washing cycles. The washed cotton fabric was washed with distilled water at 40 ℃ and dried at 60 ℃. The fluorescent whitening sample was measured using the Datacolor 800 spectrophotometer and the change curve of whiteness with washing time drawn.
The fluorescent whitening sample and a set of reference samples (grade 4 blue wool standard samples) were exposed under an artificial light source according to the prescribed conditions and then the sample and reference samples compared for color changes to evaluate color fastness. This test is based on ISO 105-B02: 2014 Method 3. As fluorescent whitening textiles usually use 3-4 level blue wool standard sample test level, the 4 level blue wool standard sample test level was used in this test.
Skin Safety Test
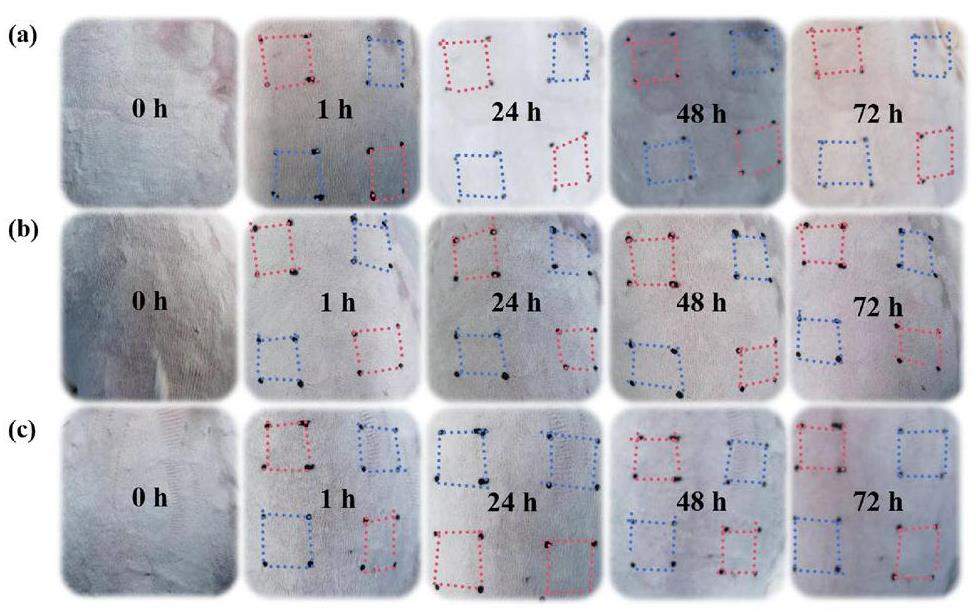
Three healthy adult female (not pregnant, not produced) New Zealand white rabbits were selected for skin safety testing. The samples are fluorescent whitening cotton fabric and blank gauze. Within 24 h before administration, hair removal was performed on both sides of an animal’s back. The hair removal area should meet the application needs and the hair removal area was about 10 cm × 15 cm (Fig. 3). The sample was cut into small pieces with an area of 2.5 cm × 2.5 cm, and the sample directly attached to the corresponding depilated skin, and then a layer of cellophane and four layers of gauze applied. Finally, medical tape was used for sealing and bandaging. After 4 h of application, the sample was removed and the administration site marked with an oily marker pen. The residual sample on the skin was washed with warm water and dried. Blank gauze was used on the control site. The operation of administration was the same as that of the sample. The erythema and edema reactions of animal skin were observed at 1 ± 0.1 h, 24 ±2 h, 48 ±2 h, and 72 ± 2 h after removing the patch. The score was evaluated according to the observation results and the primary irritation index (PII) calculated according to Eq. 2, expressed as
Results and discussion
Structural Analysis of DCH
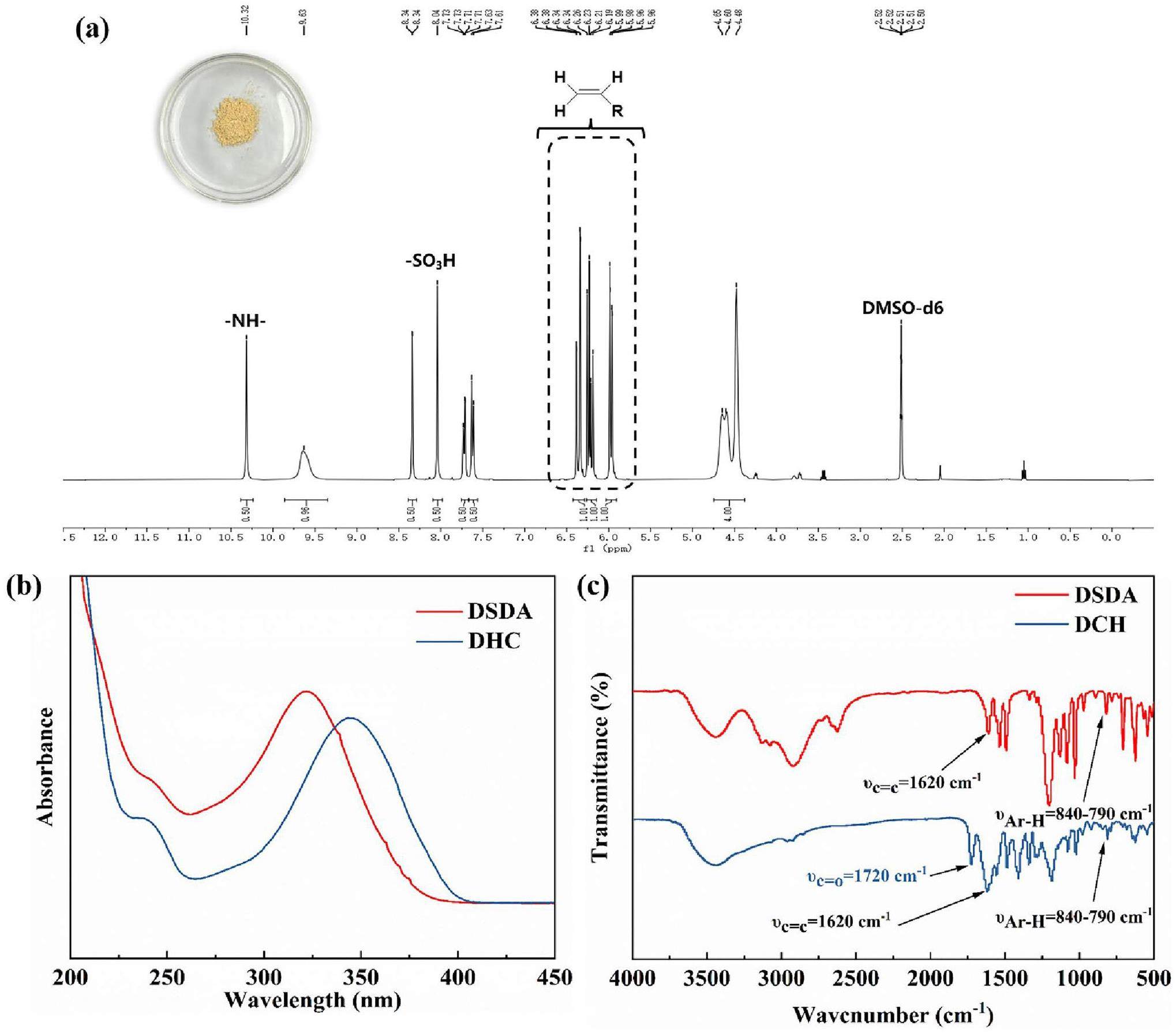
The chemical shifts of the hydrogen atoms contained in vinyl ranged from 5.8 to 6.6 in 1H-NMR, which were clearly seen (Fig. 4a). The high proportion of vinyl groups in DCH molecules was a prerequisite for high polymerization activity. The 1H-NMR (Fig. S2) and 13C-NMR (Fig.S3) of the intermediate and 13C-NMR (Fig. S4) of DCH are detailed in the Supplementary Information. From the UV absorbance spectra of DSDA and DCH, there was a weak absorbance peak in the range of 230-240 nm, which was the UV absorbance peak of C=C. The maximum absorbance wavelength of DSDA was in the range of 320-330 nm. After modification with CC and HEA, the maximum absorbance wavelength of DCH appeared at 340-350 nm, which was also the maximum absorbance wavelength range of most fluorescent whitening agent stable structures. DSDA fluorescent whitening agents usually contain cis-trans isomers, but the absorbance peak of the cis-structure of DCH was hardly observed. The reason for this might have been that HEA was long-linked to the ends of the triazine ring, such that the product had a certain spatial steric hindrance in the chemical spatial structure. This thus hindered the transformation of trans-isomer to cis-isomer and enhanced the stability of trans-isomers. Therefore, the UV absorbance intensity of trans-isomer was significantly greater than that of cis-isomer. The active component of DCH was the trans-isomer, which was fluorescent, whereas the cis-isomers were not [35]. By comparing the FT-IR spectra of DSDA and DCH, it was seen that they both had C=C stretching vibrations [36] and aromatic ring in-plane stretching vibrations [37] at 1620 cm-1 and 840-790 cm-1. However, the DCH molecule also had C=O stretching vibrations [36] at 1720 cm-1 and a S=O stretching vibrations [36] at 1290-1280 cm-1, which were characteristic functional groups in the DCH molecule.
Characteristics of whitened cotton fabric
The good whitening effect of DCH on the raw cotton fabric was clearly seen with the naked eye in daylight (Fig. 5a). Compared with the FT-IR spectrum of the raw cotton fabric (Fig. 5b), FWC-2 showed that the S=O stretching vibrations [36] appeared at 1270-1250 cm-1 and aromatic ring in-plane stretching vibrations [37] at 840-790 cm-1. The above functional groups are characteristic functional groups of the DCH structure, such that it was shown that DCH had been successfully grafted on raw cotton fabric.
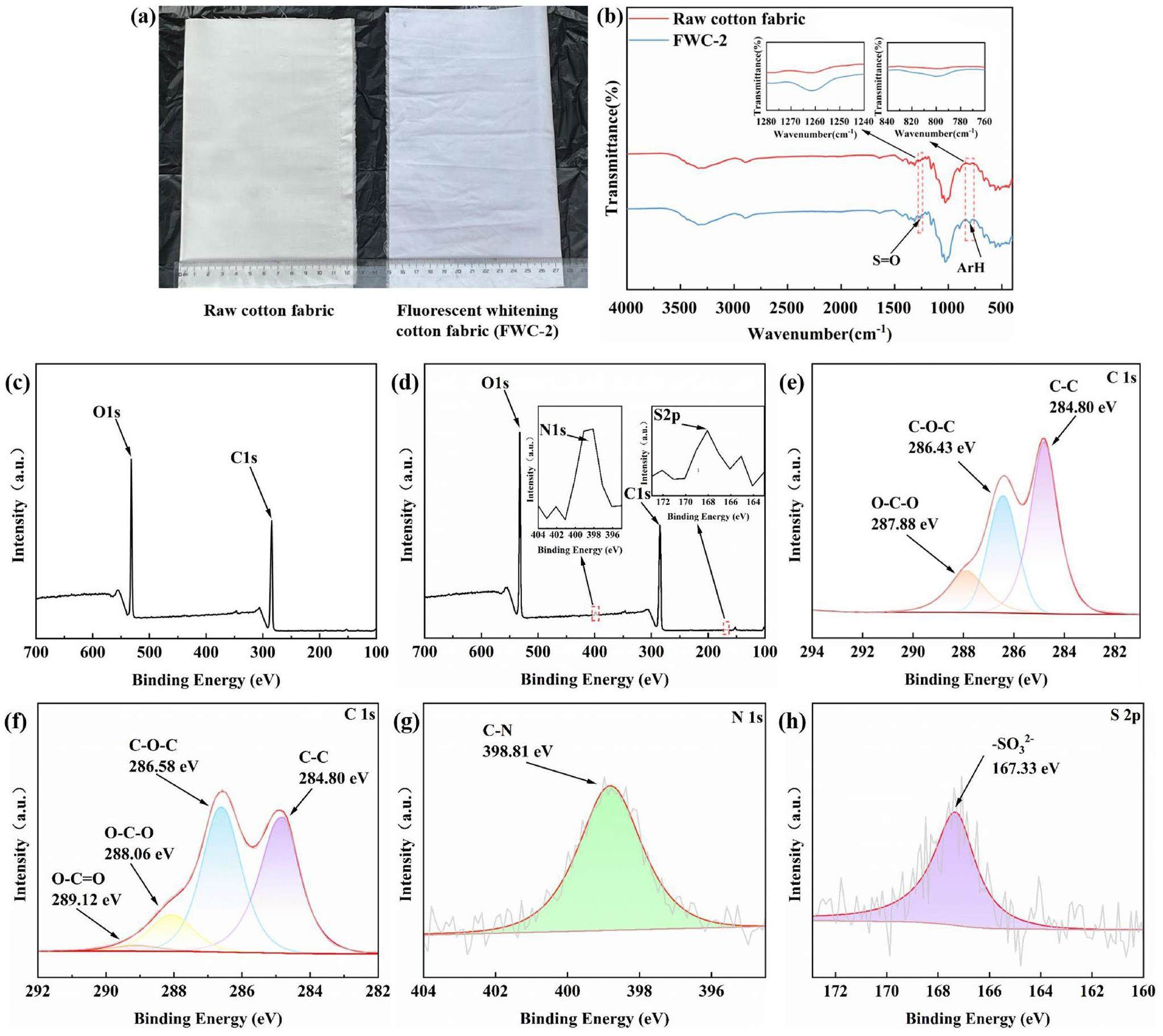
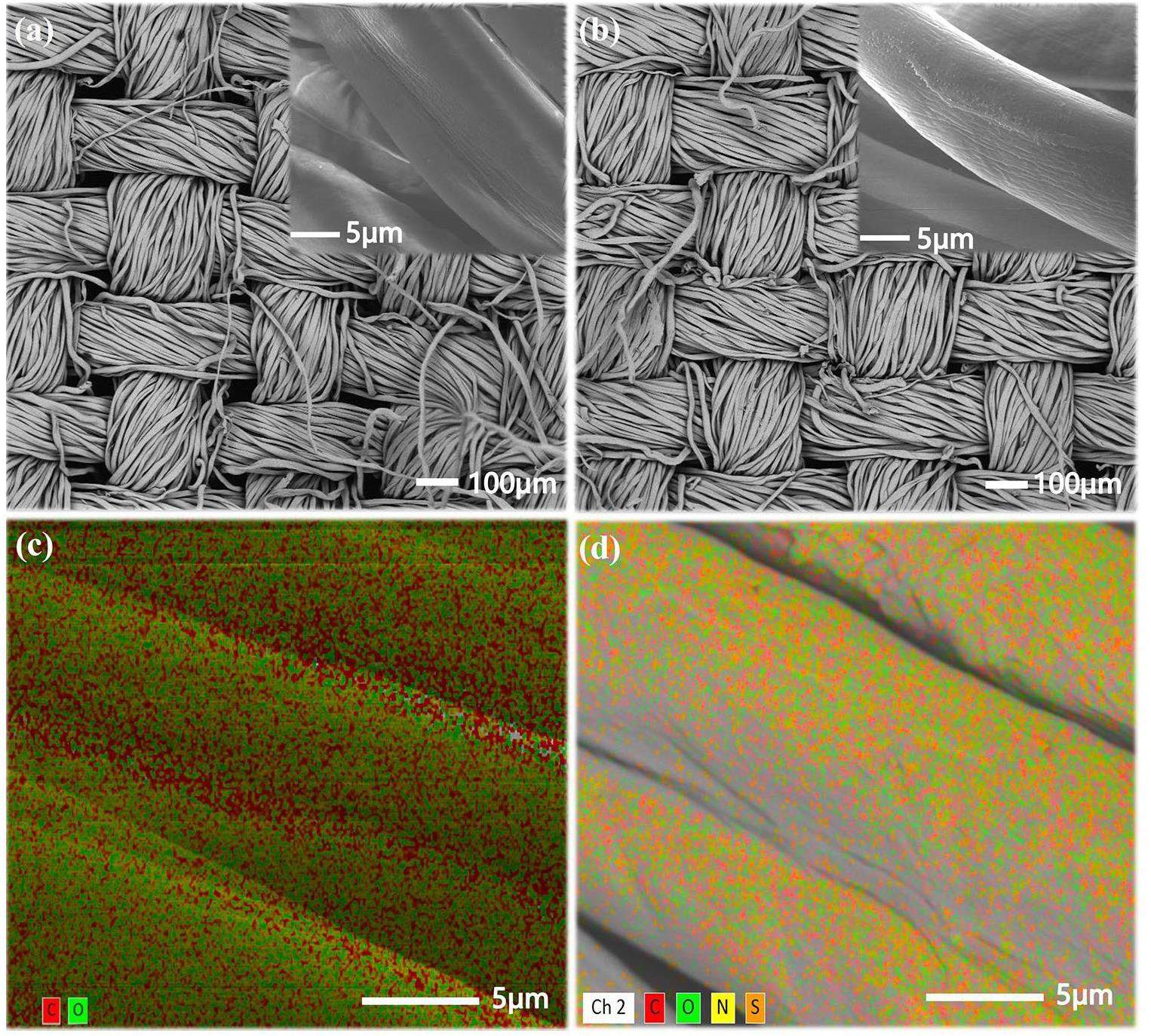
To further demonstrate that DCH was grafted on the raw cotton fabric, X-ray photoelectron spectroscopy analysis (Figs. 5c-h) was performed on the raw cotton fabric and FWC-2. Only carbon, hydrogen and oxygen were observed on the raw cotton fabric, while there were not only carbon, hydrogen, and oxygen observed in the fabric, but also nitrogen and sulfur on the treated cotton fabric. The characteristic peaks of the C-1s XPS spectrum at 284.8 eV, 286.47 eV, 288.27 eV, and 289.78 eV were attributed to C-C, C-O-C, O-C-O, and O-C=O [6]. The characteristic peak of N-1s XPS spectrum at 398.81 eV belonged to C-N [38]. The characteristic peak of S-2p XPS spectrum at 167.33 eV belonged to
Utilization of the fluorescent whitening agent
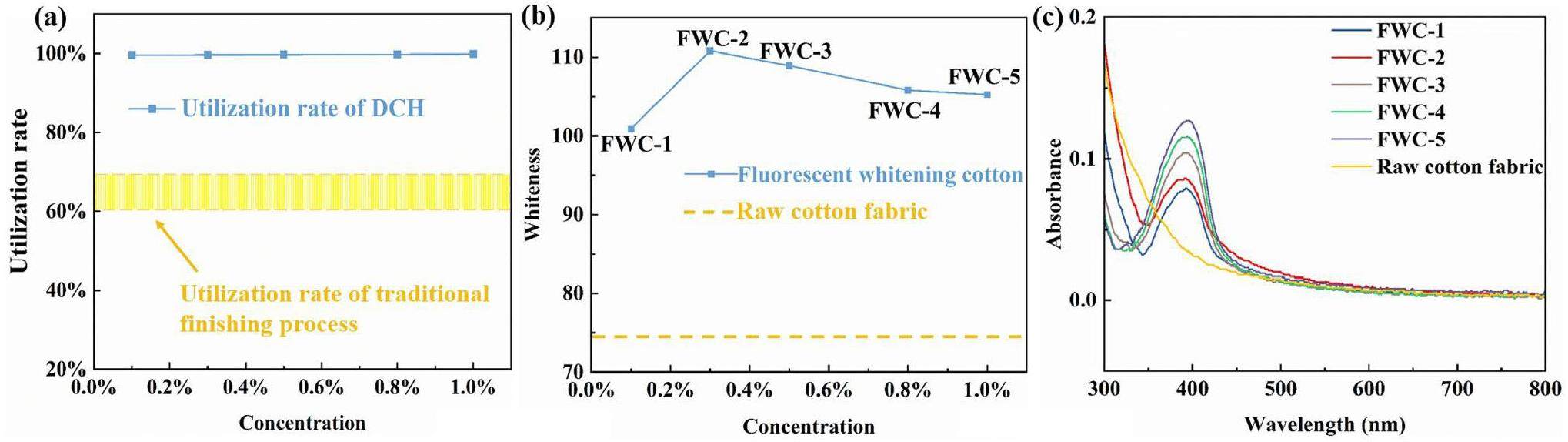
In the traditional textile dyeing and finishing process, the principle of fluorescent whitening agent for finishing raw cotton fabric is similar to that of direct dyes but that for polyester is similar disperse dye [40]. They bind to raw cotton fibers through weaker chemical bonds, such as van der Waals forces and hydrogen bonds. Therefore, this process has a low utilization rate of dyes. Compared with the utilization rate (60-70%) of traditional fluorescent whitening processes, the present the utilization rate of DCH was close to 100% using electron beam irradiation grafting technology (Fig. 7a). The whiteness of the raw cotton fabric without whitening finishing was 74.50. DCH exhibited a good whitening effect on raw cotton fabric (Fig. 7b). With increased applied DCH concentration on the fabric, the blue-purple fluorescence intensity emitted by it also increased. When the concentration of whitening agent on the fabric increased to a suitable concentration, the blue-purple fluorescence emitted an offset yellow light on the fabric and the fabric whitening effect the best. As the concentration continues to increase, its own pale yellow will appear which caused the whitening effect to decrease [41]. At low concentration, the whiteness value increased with increased DCH concentration. When the concentration was greater than 0.3 wt%, the whiteness began to decrease. Therefore, the optimal DCH concentration was 0.3 wt%, with the fluorescent whitening effect the best and whiteness value the highest (110.81). In the concentration range of 1 wt%, the whiteness values were much higher than the raw cotton fabric whiteness. Compared with the raw cotton fabric, DCH-treated cotton fabric had an absorbance peak at 390 nm and absorbance increased with increased DCH concentration (Fig. 7c). According to the UV absorbance spectrum of DCH (Fig. 4b), the maximum absorption wavelength of DCH was 350 nm.This red shift phenomenon is attributed to the interaction between cotton fabric macromolecules and fluorescent whitening agent molecules [42]. This also showed that DCH was successfully dyed onto the surface of raw cotton fabric and the blue-purple fluorescence reflected by absorbing UV light, thus offsetting the yellow color of the raw cotton fabric and finally achieving the effect of fluorescent whitening.
Washing and lighting durability
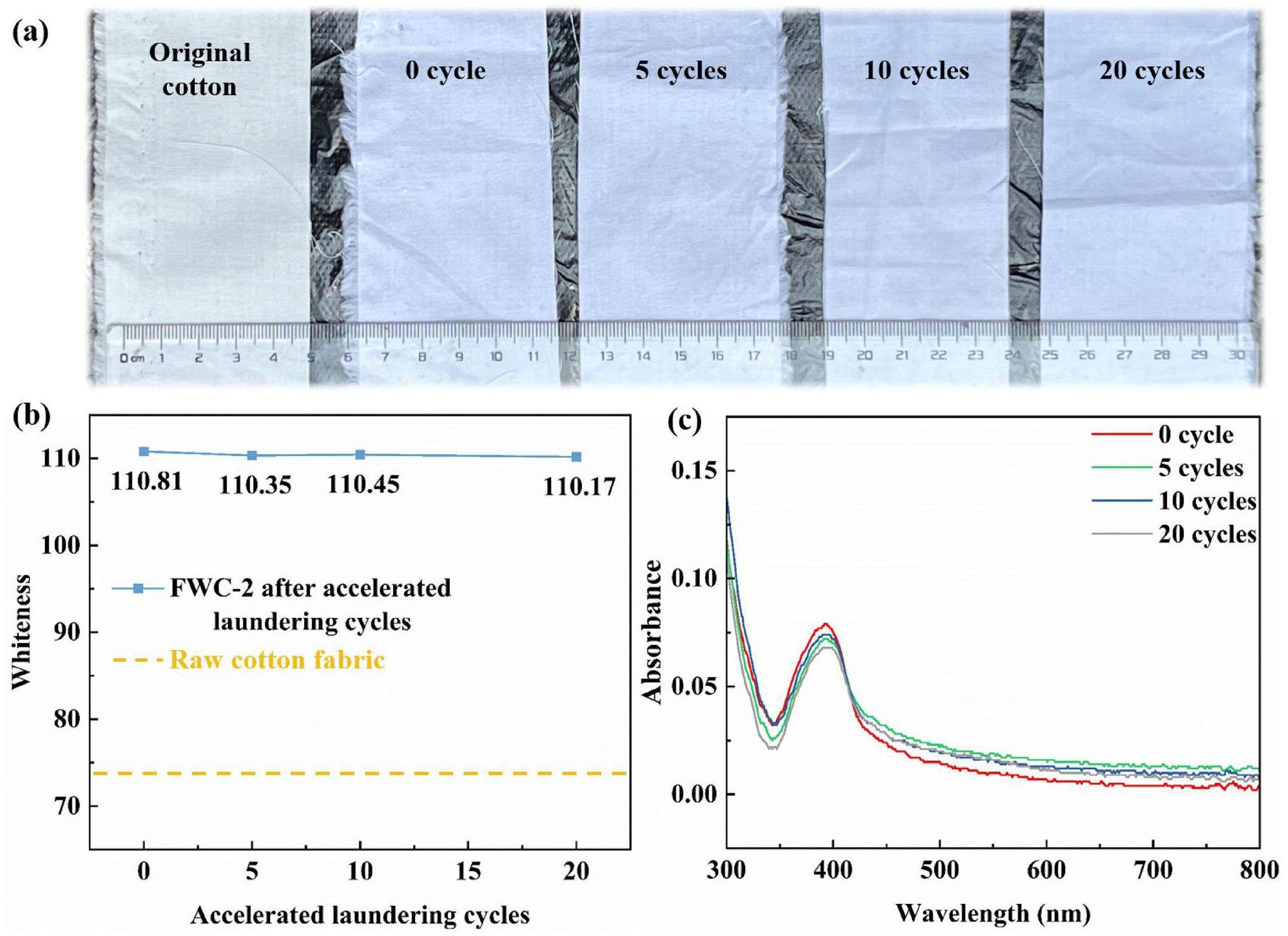
As mentioned above, traditional fluorescent whitening agents are combined with cotton fibers by weak chemical bonds, such that the finished products usually have low washing durability. However, the present fluorescent whitening finishing of cotton fabric was carried out by electron beam irradiation grafting technology, such that covalent bonds between the two gave it excellent washing fastness. According to the results of washing fastness tests of FWC-2, the change curve of whiteness with the number of accelerated laundering cycles was drawn (Fig. 8b). With increased number of accelerated laundering cycles, the whiteness of FWC-2 hardly changed (Fig. 8a). The UV-Vis absorbance spectra of FWC-2 before and after accelerated laundering cycles showed that the absorbance of washed cotton fabric did not change much before and after washing (Fig. 8c). Even under the condition of 20 accelerated laundering cycles (equivalent to 100 household washings), whiteness still remains above 110, demonstrating excellent color fastness to washing (with a color fastness grade of 5). For comparison, we used a commonly-used fluorescent whitening agent, C186 (Fig. S6) to whiten cotton fabric via the traditional finishing process. It was found that the color fastness grade of the C186 whitened cotton fabric was only 1-2 [43] (Table 2). This means that it is very easily washed away, thereby greatly reducing the fluorescent whitening effect and the migration of fluorescent whitening agents might lead to environmental pollution and biosafety risks. Under sunlight, fluorescent whitening agent molecules can produce a photoisomerization phenomenon and the planar trans-structure can be transformed into a nonplanar cis-structure, thus losing fluorescence effect. The traditional finishing process makes the fluorescent whitening agent and cotton fiber combine with weak chemical bonds, which reduces the torsion performance of the stilbene structure and inhibits the process of cis-trans isomerization. However, with increased sun exposure time, the effect of fluorescent whitening also decreases significantly [44]. Herein, the light fastness of FWC-2 was also tested and the results showed that light fastness was better than grade of 4. The light fastness of C186 whitened cotton fabric was only 1-2 [43] (Table 2). As a result, DCH also showed excellent light durability. The reason for this was that the electron beam irradiation grafting technology enabled DCH molecules to be firmly covalently bonded to the raw cotton fabric, which improved the excellent light durability of the finished cotton fabric.
| Sample | Washing durability rank | Lighting durability rank |
|---|---|---|
| FWC-2 | 5 | 4-5 |
| C186 | 1-2 | 1-2 |
Skin Safety
The combination of fluorescent whitening agent with cotton fiber by weak chemical bond also leads to its increased mobility. Therefore, the safety of a fluorescent whitening agent has always been a focus of discussion. As a fluorescent whitening agent used in textile cotton, its skin irritation experimentation is inevitable. Here, the covalently fluorescent whitening agent on the raw cotton fabric played a positive role. FWC-2 samples were used to test the skin irritation of New Zealand white rabbits. The skin response of New Zealand white rabbits was observed (Fig. 9), and the primary irritation index (PII) of all experimental groups was 0 (Table 3). No abnormal clinical symptoms except skin reaction were found in all animals, which showed that FWC-2 had no irritation to the surface of mammalian skin and met the safety standards of textiles. The specific experimental details and evaluation criteria are listed in the Supplementary Information (Table S1-S6). Here, the feasibility was shown for using DCH for fluorescent whitening finishing of cotton fabric by electron beam irradiation grafting technology and also provided an important basis for its industrialization.
| Animal No. | Dosing zone | Skin reaction | Interval (hours) 1±0.1 | 24±2 | 48±2 | 72±2 | Average score |
|---|---|---|---|---|---|---|---|
| 1 | Test site (Left) | Erythema | 0 | 0 | 0 | 0 | 0 |
| Edema | 0 | 0 | 0 | 0 | |||
| Test site (Right) | Erythema | 0 | 0 | 0 | 0 | ||
| Edema | 0 | 0 | 0 | 0 | |||
| Negative site (Left) | Erythema | 0 | 0 | 0 | 0 | 0 | |
| Edema | 0 | 0 | 0 | 0 | |||
| Negative site (Right) | Erythema | 0 | 0 | 0 | 0 | ||
| Edema | 0 | 0 | 0 | 0 | |||
| 2 | Test site (Left) | Erythema | 0 | 0 | 0 | 0 | 0 |
| Edema | 0 | 0 | 0 | 0 | |||
| Test Site (Right) | Erythema | 0 | 0 | 0 | 0 | ||
| Edema | 0 | 0 | 0 | 0 | |||
| Negative Site (Left) | Erythema | 0 | 0 | 0 | 0 | 0 | |
| Edema | 0 | 0 | 0 | 0 | |||
| Negative site (Right) | Erythema | 0 | 0 | 0 | 0 | ||
| Edema | 0 | 0 | 0 | 0 | |||
| 3 | Test site (Left) | Erythema | 0 | 0 | 0 | 0 | 0 |
| Edema | 0 | 0 | 0 | 0 | |||
| Test site (Right) | Erythema | 0 | 0 | 0 | 0 | ||
| Edema | 0 | 0 | 0 | 0 | |||
| Negative site (Left) | Erythema | 0 | 0 | 0 | 0 | 0 | |
| Edema | 0 | 0 | 0 | 0 | |||
| Negative site (Right) | Erythema | 0 | 0 | 0 | 0 | ||
| Edema | 0 | 0 | 0 | 0 |
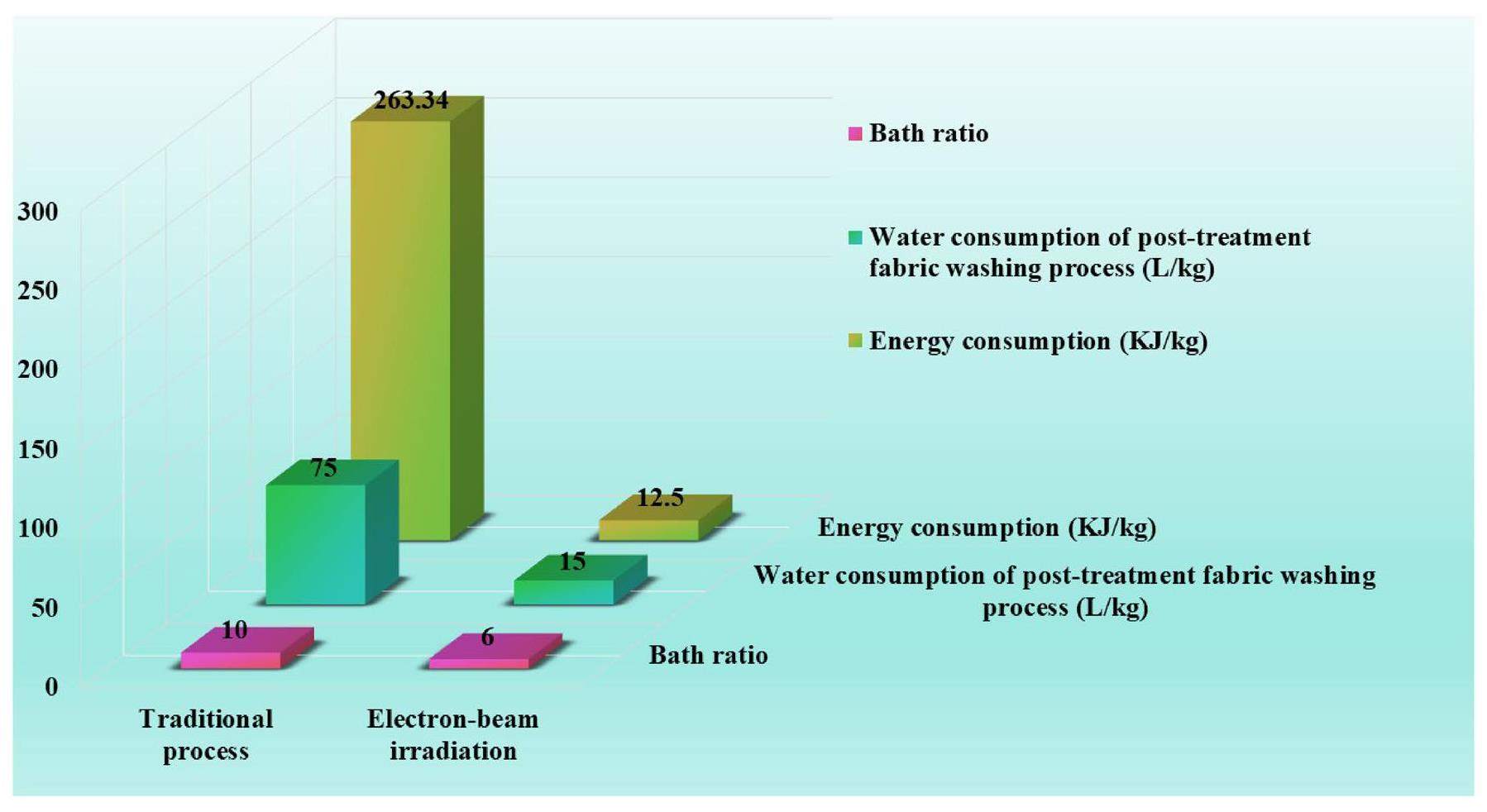
Energy and water consumption
In the process of fluorescent whitening via electron beam irradiation, the consumption of energy is almost exclusively generated by electron accelerator devices. The dose of 10 kGy meant that 1 kg of irradiated material absorbs 10 kJ of energy and the energy utilization rate of the electron accelerator device is not less than 80% [28]. The energy consumption of the new method was calculated based on the absorbed dose and energy utilization rate of the electron accelerator device and found to be 12.5 kJ kg-1. A simple calculation for the energy consumption of traditional finishing of 1 kg of cotton fabric was made, taking into account the energy required for heating water and maintaining the finishing vat temperature. Assuming the need to heat water from 20 ℃ to the typical process temperature of 50 ℃ and the specific heat capacity of water as 4.18 J g-1℃-1, the energy Q required to heat 1 kg of water was calculated using Eq. 3
| Sample | COD (mg l-1) | Electrical conductivity (μS cm-1) |
|---|---|---|
| Deionized water | 0 | 0 |
| Wastewater of FWC-2 | 22 | 56 |
| Wastewater of traditional finishing process | 5428 | 2010 |
Conclusion
In this study, a vinyl-containing fluorescent whitening agent was synthesized and used to whiten raw cotton fabric, achieving about 100% of utilization rate assisted by electron beam irradiation. The vinyl-containing fluorescent whitening agent was confirmed to be covalently grafted onto the surface of cotton fibers. After treatment, the whiteness value of fabric reached 110.81 from the 74.50 of the original fabric using the present whitening solution at 0.3-wt% concentration. Due to covalent bonding with fibers, the whitened fabric maintained 110+ of whiteness value, even after the equivalent of 100 domestic washing cycles. This also exhibited excellent light fastness with a grade of 4, demonstrating the good washing and lighting durability of the fluorescent whitening effect produced via electron beam irradiation. Skin irritation tests showed the whitened fabric caused 0 of the primary irritation index (PII) and no abnormal clinical symptoms, demonstrating its outstanding skin safety. The energy and water consumption of this process were also analyzed and found to require energy only 125 kJ kg-1 raw cotton fabric, which was much lower than the 263.34 kJ kg-1 required by the traditional process. The finishing wastewater generated from this process could save at least 180 L kg-1 of cotton fabric. In addition, the spent washing water of the above finishing process had such a low COD and electrical conductivity that they completely met the direct discharge of the wastewater, substantiating the energy conservation and pollution reduction characteristics of the electron beam irradiation induced whitening method. All in all, this new fluorescence whitening finishing process via electron beam irradiation provided a green and sustainable pathway for development of the textile industry.
Surface nano-engineering of cellulosic textiles for superior biocidal performance and effective bacterial detection
. Chem. Eng. J. 473,Environmentally friendly dyeing of cotton in an ethanol-water mixture with excellent exhaustion
. Green Chem. 20, 4473-4483 (2018). https://doi.org/10.1039/C8GC01814FNovel amino acid-stilbene quaternary ammonium salt fluorescent whitening agents: synthesis, optical properties, acid resistance and anti‐bacterial activity
. Color. Technol. 138, 201-209 (2022). https://doi.org/10.1111/cote.12584Synthesis and application of bioadsorbent for Y(III) ions from Poly(vinyl amine) grafted onto rayon by radiation-induced grafting
. Nucl. Eng. Technol. 57,A review on treatment technologies for printing and dyeing wastewater (PDW)
. J. Water Process. Eng. 50,Fabrication of multifunctional cotton fabrics with antibacterial, hydrophobic, and dyeing performance
. ACS Appl. Mater. Interfaces. 15, 51727-51736 (2023). https://doi.org/10.1021/acsami.3c10852Identification and trend analysis of organic cationic contaminants via non-target screening in suspended particulate matter of the German rivers Rhine and Saar
. Water Res. 229,Respirometric study of optical brighteners in textile wastewater
. Materials 12, 785 (2019). https://doi.org/10.3390/ma12050785Fluorescent whitening agents in Baiyangdian Lake in North China: Analysis, occurrence, distribution and ecological risk assessment
. Environ. Pollut. 291,Eco-friendly dyeing of raw cotton fibres in an ethanol-water mixture without scouring and bleaching pretreatments
. Green Chem. 23, 796-807 (2021). https://doi.org/10.1039/D0GC02839HCharacterization of carbon fiber and glass fiber reinforced polycarbonate composites and their behavior under gamma irradiation
. Prog. Nucl. Energy. 134,Effect of gamma irradiation on the mechanical behavior, thermal properties and structure of epoxy/glass-fiber composite
. J. Nucl. Mater. 441, 67-72 (2013). https://doi.org/10.1016/j.jnucmat.2013.05.041Effect of γ irradiation on the properties of basalt fiber reinforced epoxy resin matrix composite
. J. Nucl. Mater. 466, 100-107 (2015). https://doi.org/10.1016/j.jnucmat.2015.07.037Enhancement of flame retardancy and mechanical properties of HDPE/EPM based radiation shielding composites by electron beam irradiation
. J. Nucl. Mater. 429, 99-104 (2012). https://doi.org/10.1016/j.jnucmat.2012.05.036Gamma radiation induced tailoring the structural, optical, surface and mechanical properties of UHMWPE
. Prog. Nucl. Energy. 177,The effect of gamma ray irradiation on PAN-based intermediate modulus carbon fibers
. J. Nucl. Mater. 443, 26-31 (2013). https://doi.org/10.1016/j.jnucmat.2013.06.049Application of electron beam irradiation for the removal of dye from textile effluents: A review
. Ind. Eng. Chem. Res. 63, 9611-9618 (2024). https://doi.org/10.1021/acs.iecr.4c00072Radiation induced graft polymerization of a fluorinated acrylate onto fabric
. Radiat. Phys. Chem. 81, 1354-1356 (2012). https://doi.org/10.1016/j.radphyschem.2011.11.050Preparation of dynamic superhydrophobic cotton fabric via radiation-induced graft polymerization
. Acta Polym. Sin. Volume 149, 315-320 (2017). https://doi.org/10.11777/j.issn1000-3304.2017.16273 (in Chinese)Optimization of synthesis conditions for preparation of radiation grafted polymeric fibers and process variables of adsorption with response surface methodology
. Prog. Nucl. Energy. 155,Radiation grafted cellulose fabric as reusable anionic adsorbent: A novel strategy for potential large-scale dye wastewater remediation
. Carbohydr. Polym. 249,A mild method of amine-type adsorbents syntheses with emulsion graft polymerization of glycidyl methacrylate on polyethylene non-woven fabric by pre-irradiation
. Radiat. Phys. Chem. 81, 1393-1397 (2012). https://doi.org/10.1016/j.radphyschem.2011.11.042Recent progress in environmental applications of functional adsorbent prepared by radiation techniques: A review
. J. Hazard. Mater. 424,Preparation of anti-static textile with covalently grafted polyaniline by a three-step process
. J. Radiat. Res. Radiat. Proc. 33, 28-33 (2015). https://doi.org/10.11889/j.1000-3436.2015.rrj.33.040302 (in Chinese)Covalently doping polyaniline-based photothermal fabric for continuous recovery of salt and freshwater from seawater via solar-driven interfacial evaporation
. Desalination 580,Manufacturing robust MXene-based hydrogel-coated cotton fabric via electron-beam irradiation for efficient interfacial solar evaporation
. Chem. Eng. J. 473,Non-effluent dyeing for cotton fabric at room temperature usingradiation-induced graft polymerization technology
. Textile Dyeing and Finishing Journal. 42, 13-16 (2020). (in Chinese)Greenly and efficiently dyeing cotton fabric with custom-tailored reactive dyes via electron beam irradiation
. ACS Sustain. Chem. Eng. 12, 3121-3129 (2024). https://doi.org/10.1021/acssuschemeng.3c07075Radiation grafting of cotton fabric for "green" dyeing with acid dyes
. Journal of Radiation Research and Radiation Processing. 40, 25-31 (2022). https://doi.org/10.11889/j.1000-3436.2021-0028 (in Chinese)"Green" dyeing of cotton fabric by radiation-initiated immobilizing nanoparticles
. Journal of Radiation Research and Radiation Processing. 38, 63-66 (2020). https://doi.org/10.11889/j.1000-3436.2020.rrj.38.011001 (in Chinese)A promising clean way to textile colouration: cotton fabric covalently-bonded with carbon black, cobalt blue, cobalt green, and iron oxide red nanoparticles
. Green Chem. 21, 6611-6621 (2019). https://doi.org/10.1039/C9GC02084ESuperhydrophobic self-extinguishing cotton fabrics for electromagnetic interference shielding and human motion detection
. J. Mater. Sci. Technol. 132, 59-68 (2023). https://doi.org/10.1016/j.jmst.2022.05.036Hydrophobic silicone modified membranes for efficient oil/water separation: Synthesis, fabrication and application
. Sep. Purif. Technol. 353,Photochemistry of some polymerizable fluorescent brighteners
. J. Photoch. Photobio. A 135, 41-44 (2000). https://doi.org/10.1016/S1010-6030(00)00276-8The synthesis and properties of some triazine-stilbene fluorescent brighteners
. Dyes Pigment. No. 2, 155-160 (1995). https://doi.org/10.1016/0143-7208(95)00040-MSynthesis and properties of amphoteric fluorescent whitening agents
. Journal of Shandong University (Engineering Science). 40, 108-112 (2010). (in Chinese)Regulating the alkyl chain length of quaternary ammonium salt to enhance the inkjet printing performance on cationic cotton fabric with reactive dye ink
. ACS Appl. Mater. Interfaces. 15, 19750-19760 (2023). https://doi.org/10.1021/acsami.3c02304The effect of the number of sulfonic acids in polysiloxanes as fillers for polymer electrolyte membranes in energy storage applications
. Colloid. Surface. A 695,The optimization of whiteness of polyester fabric treated with nanoparticles of 2,2’-(vinylenedi-p-phenylene)bis-benzoxazole (OB-1) by the Taguchi method
. Colloid. Surface. A 676,Technological development of fluorescent brightener and its impact on the environment (to be continued)
. Textile Auxiliaries. 32, 1-10 (2015). (in Chinese)Spectroscopic Studies of the Intermolecular Interactions of Congo Red and Tinopal CBS with Modified Cellulose Fibers
. Langmuir 21, 5414-5420 (2005). https://doi.org/10.1021/la046842jThe synthesis and properties of triazine-stilbene fluorescent brighteners containing a monophenolic antioxidant
. Dyes Pigment. 75, 681-686 (2007). https://doi.org/10.1016/j.dyepig.2006.07.018Photophysical and photochemical properties of some triazine-stilbene fluorescent brighteners
. Dyes and Pigments. 44, 175-180 (2000). https://doi.org/10.1016/S0143-7208(99)00088-1A review of energy use and energy efficiency technologies for the textile industry
. Renew. Sust. Energ. Revi. 16, 3648-3665 (2012). https://doi.org/10.1016/j.rser.2012.03.029The online version contains supplementary material available at https://doi.org/10.1007/s41365-025-01732-1.

